Sarah Crome, PhD Associate Professor | University of Toronto | Faculty of Medicine | Department of Immunology Senior Scientist | University Health Network | Toronto General Hospital Research Institute | Multi Organ Transplant Tier 2 Canada Research Chair in Tissue-Specific Immune Tolerance
Dr. Sarah Crome established her lab at University Health Network (UHN) and the University of Toronto in 2018. She holds a Tier 2 Canada Research Chair in Tissue-Specific Immune Tolerance. Her expertise is in human immunology, immune tolerance and immunotherapy, with a particular focus on innate lymphoid cell family members and CD4+ T helper cells. Her research harnesses the latest single cell and spatial technologies to map cellular circuits including NK cells and other ILC family members in human health and disease, as well as advanced immunological and genetic platforms to study human lymphocyte biology. Her translational research involves the identification of novel immunotherapeutic targets and development of cell-based therapies,
Dr Crome’s scientific career started at the University of British Columbia with Dr. Megan Levings, where her research defined molecular, epigenetic and cellular regulatory mechanism that control human CD4+ T helper 17 cell development and their pro-inflammatory functions. Her postdoctoral work with Dr. Pamela Ohashi at Princess Margaret Cancer Centre discovered a novel innate lymphoid cell (ILC) population that inhibits the activity and expansion of tumour-associated T cells and is associated with poorer clinical outcomes in ovarian cancer. Dr. Crome has received the Rising Star award from the International Union of Immunological Societies, the Next Generation of Scientists award from the Cancer Research Society, and the New Investigator award from Canadian Society for Immunology.
Contact: email: sarah.crome[at]utoronto.ca twitter: @sarah_crome bluesky: @sarahcrome.bsky.social phone: 416.634.8097 address: 2-805 PMCRT | 101 College St | Toronto ON M5G 1L7 | Canada
Websites with additional information: https://www.immunology.utoronto.ca/faculty/sarah-crome https://www.uhnresearch.ca/researcher/sarah-q-crome
Current Lab Members:
Graduate Students
Kyle Reid (PhD Student) Julia Murphy (PhD Student)
Sarah Colpitts, MSc (PhD Student)
Sinthuja Jegatheeswaran, MSc (PhD Student)
Martin Mak (PhD Student)
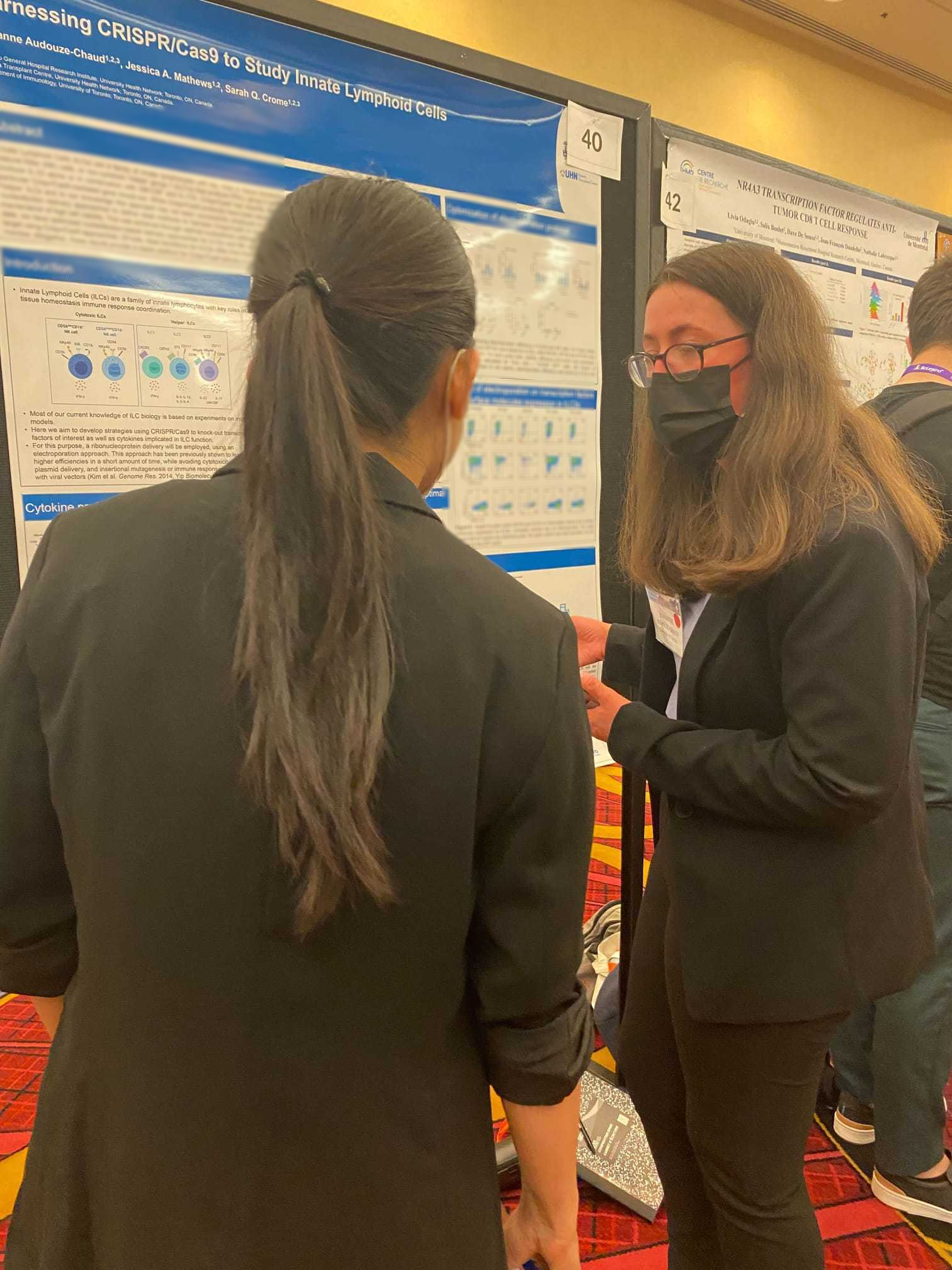
Johanne Audouze (MSc Student)
Sia Mashhouri (PhD Student)
David Luong (PhD Student, co-supervised)
Staff
Humaira Murshed
Nadia Sachewsky, PhD (Microsurgeon)
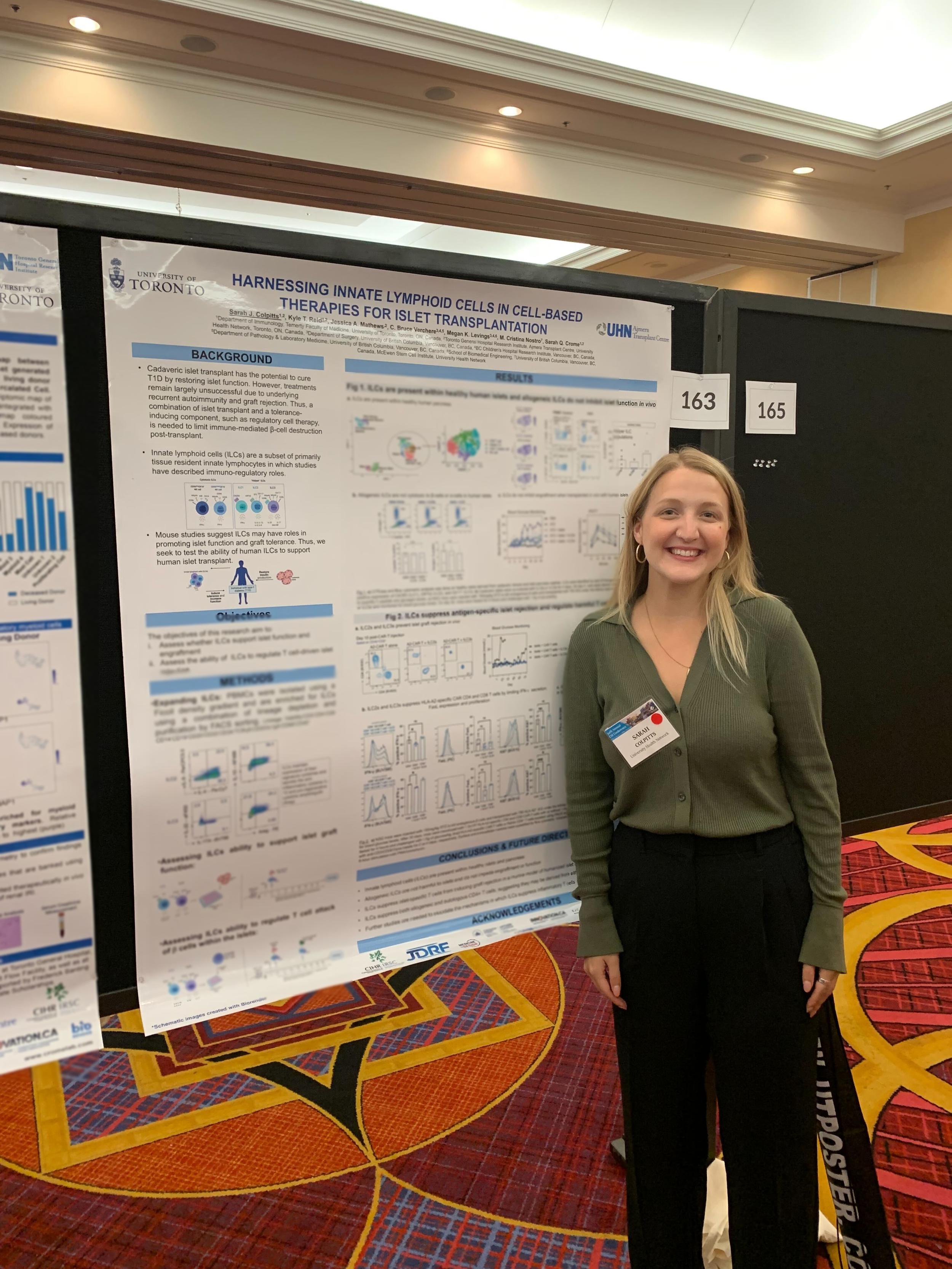
Sarah Colpitts
Sarah is characterizing the role of innate lymphoid cells in type 1 diabetes (T1D). T1D is an autoimmune disease in which inflammatory CD4+ T cells attack against pancreatic islet β-cells, which secrete insulin and facilitate the break-down of glucose. She is exploring how the tissue regenerative and regulatory properties of ILCs influence effectiveness of islet transplantation, and how ILCs are altered in T1D.
KYLE REID
Development of graft-versus-host disease (GVHD) is the primary factor limiting the success of hematopoietic stem cell transplant (HSCT) in treating hematological malignancies. Kyle is focused on determining if and how different ILC subsets contribute to protection from the development of GVHD. He is combining single cell RNA sequencing of patients undergoing HSCT, humanized mouse models of GVHD and advanced immunological platforms to understand ILC interactions that are protective in these contexts.
JULIA Murphy
Julia is investigating the role of human innate lymphoid cells (ILCs) and other tissue-resident immune cells in kidney transplantation. She harnesses multi-omic single-cell approaches and functional assays to identify and characterize ILCs in kidney from healthy living donors and transplant recipients to identify immune cells and pathways that impact clinical outcomes in kidney transplant recipients.
MARTIN MAK
Martin is focusing on the role of tissue resident immune cells in kidney transplantation, with the aim of determining what causes recipients of deceased kidney allografts to exhibit worse clinical outcomes than recipients of living kidney allografts. Martin makes use of multi-omic single cell techniques to focus on immune cell differences between living and deceased donor kidneys, which will be combined with our cell and tissue culture models to reveal host and donor immune differences.
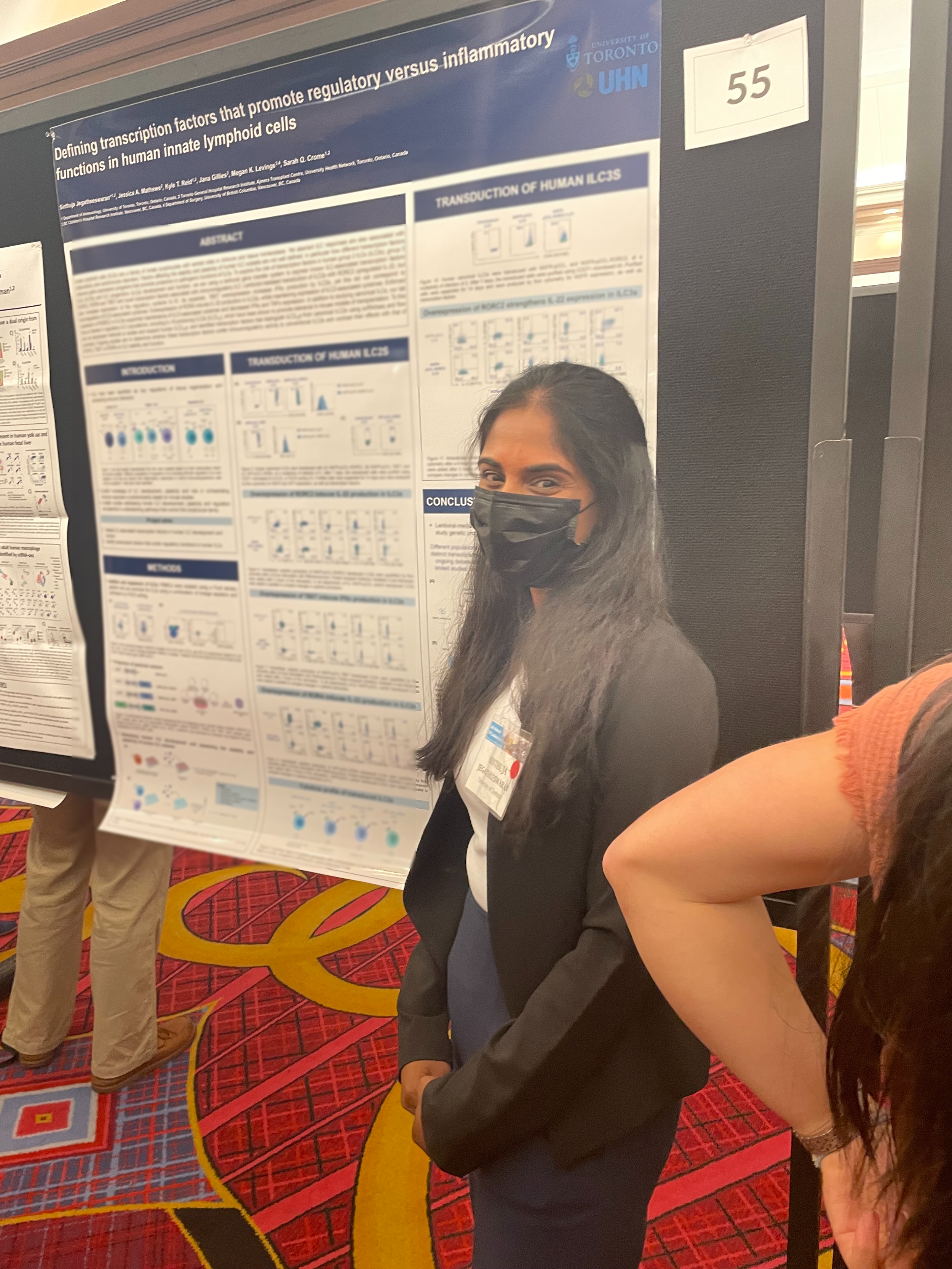
SINTHUJA JEGATHEESWARAN
Sinthuja is investigating the interaction between human innate lymphoid cells (ILCs) and regulatory T cells (Tregs), and its cell therapy potential in the context of type 1 diabetes (T1D) and islet transplantation. She is exploring the ability of ILCs, in combination with Tregs, to promote tolerance and function of transplanted beta-like cells. She will also harness lentiviral vector-based systems to investigate the role of ILC associated transcription factors on human ILC development and function, and explore the potential of different transcription factors to confer immunoregulatory function of ILCs.
Siavash Mashhouri
Siavash’s research delves into the role of innate lymphoid cells (ILCs) within transplant immunology, with a specific focus on hematopoietic stem cell transplantation (HSCT) and graft-versus-host disease (GVHD). Through the use of cutting-edge technological platforms, he is exploring the biology of human ILCs in xenogenic GVHD, with the central aim being to leverage the protective functions of ILCs to create novel cell-based therapies. By engineering immune cells, he strive to optimize current immunotherapies and advance treatment outcomes for transplant patients.
Sebastian Grocott
Sebastian is focused on understanding factors that regulate human adaptive Natural Killer (NK) cells, innate lymphocytes with anti-viral memory-like properties, in peripheral tissues following stem cell and solid organ transplantation. He employs single-cell RNA sequencing of transplant recipient tissues, in combination with in vitro 3D models of organ transplantation, to dissect local mechanisms tuning the functions of NK cells. He is interested in the development of targeted tissue-specific therapies for promoting tolerance in transplantation, and therefore limiting the risks of infection for systemically immunosuppressed transplant recipients
Lab Alumni:
Johanne Audouze, MSc
James An, MSc
Dorota Borovsky, MSc
Sofija Bekarovska, MSc